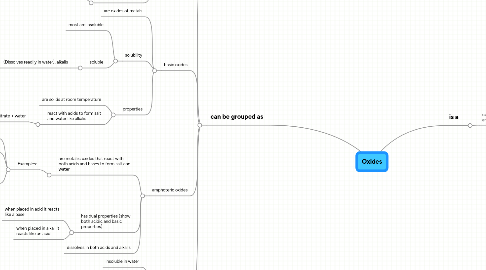
1. can be grouped as
1.1. acidic oxides
1.1.1. are oxides of non metals
1.1.2. most dissolve in water to form an acid
1.1.3. Examples of Acidic Oxides and acids produced in water:
1.1.3.1. Sulfur Dioxide SO₂Forms Sulfurous Acid (H₂SO₃)
1.1.3.2. Carbon Dioxide CO₂Forms Carbonic Acid (H₂CO₃)
1.1.3.3. Sulfur Trioxide SO₃Forms Sulfuric Acid (H₂SO₄)
1.1.4. react with alkalis to form a salt and water (like acids)
1.1.4.1. Eg: sulfur trioxide + sodium hydroxide --> sodium sulfate + water
1.2. basic oxides
1.2.1. are oxides of metals
1.2.2. solubility
1.2.2.1. most are insoluble
1.2.2.2. soluble
1.2.2.2.1. (Dissolves readily in water), alkalis
1.2.3. properties
1.2.3.1. are solids at room temperature
1.2.3.2. react with acids to form salt and water like alkalis
1.2.3.2.1. Eg: Calcium oxide + Nitric Acid --> calcium nitrate + water
1.3. amphoteric oxides
1.3.1. are metallic oxides that react with both acids and bases to form salt and water
1.3.1.1. Examples:
1.3.1.1.1. Aluminium Oxide
1.3.1.1.2. Lead (II) Oxide
1.3.1.1.3. Zinc Oxide
1.3.2. has dual properties (show both acidic and basic properties)
1.3.2.1. when placed in acid it reacts like a base
1.3.2.2. when placed in alkali it reacts like an acid
1.3.3. dissolves in both acids and alkalis
1.4. neutral oxides
1.4.1. insoluble in water
1.4.2. show neither basic nor acidic properties
1.4.3. Examples:
1.4.3.1. water
1.4.3.2. carbon monoxide (CO)
1.4.3.3. nitric oxide (NO)
