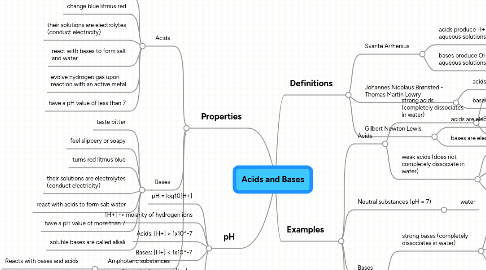
1. Properties
1.1. Acids
1.1.1. taste sour (Fun fact: the word 'acid' comes from the Latin word 'acere' which means sour)
1.1.2. change blue litmus red
1.1.3. their solutions are electrolytes (conduct electricity)
1.1.4. react with bases to form salt and water
1.1.5. evolve hydrogen gas upon reaction with an active metal
1.1.6. have a pH value of less than 7
1.2. Bases
1.2.1. taste bitter
1.2.2. feel slippery or soapy
1.2.3. turns red litmus blue
1.2.4. their solutions are electrolytes (conduct electricity)
1.2.5. react with acids to form salt water
1.2.6. have a pH value of more than 7
1.2.7. soluble bases are called alkali
1.3. Amphoteric substances
1.3.1. Reacts with bases and acids
2. pH
2.1. pH = log10[H+]
2.2. [H+] -> molarity of hydrogen ions
2.3. Acids: [H+] > 1x10^-7
2.4. Bases: [H+] < 1x10^-7
2.5. Neutral substances: [H+] = [OH-] = 1x10^-7
3. Definitions
3.1. Svante Arrhenius
3.1.1. acids produce H+ ions in aqueous solutions
3.1.2. bases produce OH- ions in aqueous solutions
3.2. Johannes Nicolaus Brønsted - Thomas Martin Lowry
3.2.1. acids are proton donors
3.2.2. bases are proton acceptors
3.3. Gilbert Newton Lewis
3.3.1. acids are electron pair acceptors
3.3.2. bases are electron pair donors
4. Examples
4.1. Acids
4.1.1. strong acids (completely dissociates in water)
4.1.1.1. sulphuric acid
4.1.1.2. nitric acid
4.1.1.3. hydrochloric acid
4.1.2. weak acids (does not completely dissociate in water)
4.1.2.1. vinegar (acetic acid)
4.1.2.2. lactic acid
4.1.2.3. citric acid from fruits like lemon
4.2. Neutral substances (pH = 7)
4.2.1. water
4.3. Bases
4.3.1. strong bases (completely dissociates in water)
4.3.1.1. sodium hydroxide
4.3.1.2. calcium hydroxide (limewater)
4.3.1.3. potassium hydroxide
4.3.2. weak bases (does not completely dissociate in water)
4.3.2.1. detergents
4.3.2.2. soap
4.3.2.3. aqueous ammonia
4.4. Amphoteric substances
4.4.1. zinc oxide
4.4.2. aluminium hydroxide
4.4.3. lead oxide
