Clinical Research Coordinator
by Cassie McDonald
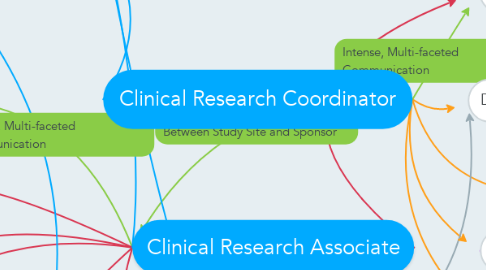
1. Study Manager
2. Clinical Research Associate
3. Study Monitor
4. Regulatory Affairs Associate
5. Safety Associates
6. Biostatisticians
7. Data Safety Monitoring Board
8. Medical Writers
9. Sponsor
10. Regulatory Bodies
11. The blue lines indicate just written communication in the form of submissions and administrative forms.
12. The purple lines indicate communication that happens regularly within a certain site or sponsor either verbally or written down. These people will also communicate with others in their site but less frequently
13. Study Site
14. Investigator
15. Clinical Research Assistant
16. Data Manager
17. Research Nurse
18. Research Pharmacist
19. The red lines (communication with the CRA) and orange lines (communication with the CRC) indicate both in-person verbal communication and written communication over emails where the information is then shared with the leaders of the respective sites of the clinical trial (sponsor and study sites).
20. The green lines indicate important and critical communication between the leaders of the study and sites and the CRAs and CRCs. These are integral communication lines and can be used either verbally (over the phone or in person) or written over email or memos.


