How does the Mentos and Diet Coke eruption occur
저자: Checkthis out
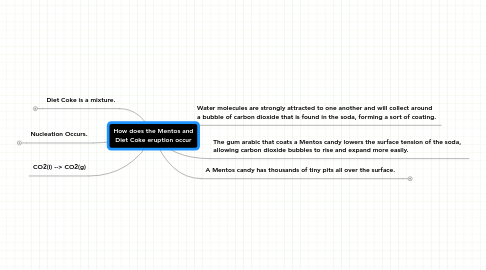

1. Diet Coke is a mixture.
1.1. Carbon dioxide [CO2] is pumped into bottles at the bottling factory using tons of pressure.
1.2. Diet Coke is super-saturated with carbon dioxide.
2. CO2(l) --> CO2(g)
3. Nucleation Occurs.
3.1. Tones of CO2 molecules are released from the liquid.
4. The gum arabic that coats a Mentos candy lowers the surface tension of the soda, allowing carbon dioxide bubbles to rise and expand more easily.
5. Water molecules are strongly attracted to one another and will collect around a bubble of carbon dioxide that is found in the soda, forming a sort of coating.
6. A Mentos candy has thousands of tiny pits all over the surface.
6.1. These pits act as nucleation sites.
6.1.1. Drop a Mentos candy into the soda, and a large number of bubbles will form very fast all over the candy at the nucleation sites.
6.1.2. As the candy sinks, it passes through more soda and creates more bubbles. Collectively, these bubbles create foam and the pressure necessary to cause an eruption.
