Inter Molecular Forces (IMF)
by M wl
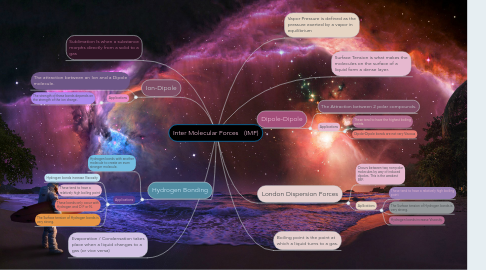

1. Ion-Dipole
1.1. The attraction between an Ion and a Dipole molecule.
1.2. Applications
1.2.1. The strength of these bonds depends on the strength of the ion charge.
2. Hydrogen Bonding
2.1. Hydrogen bonds with another molecule to create an even stronger molecule.
2.2. Applications
2.2.1. Hydrogen bonds increase Viscosity.
2.2.2. These tend to have a relatively high boiling point
2.2.3. These bonds only occur with Hydrogen and O F or N.
2.2.4. The Surface tension of Hydrogen bonds is very strong.
3. Sublimation Is when a substance morphs directly from a solid to a gas
4. Evaporation / Condensation takes place when a liquid changes to a gas (or vice versa)
5. Dipole-Dipole
5.1. The Attraction between 2 polar compounds.
5.2. Applications
5.2.1. These tend to have the highest boiling points.
5.2.2. Dipole-Dipole bonds are not very Viscous
6. London Dispersion Forces
6.1. Occurs between two non-polar molecules by way of induced dipoles. This is the weakest IMF.
6.2. Apllications
6.2.1. These tend to have a relatively high boiling point
6.2.2. The Surface tension of Hydrogen bonds is very strong.
6.2.3. Hydrogen bonds increase Viscosity.
