Chapter 3: Introductory Nuclear Physics
par Dern The Bu
1. Energy that holds a nucleus together
1.1. Stronger the binding energy per nucleon, the more stable the nucleus.
2. Nuclear Fission
2.1. Heavy Parent Nucleus splits into two daughter nuclei
2.2. Combined mass of daughter nuclei < Mass of unstable parent nucleus
2.2.1. E = mc^2
2.3. Reproduction constant K - average number of neutrons that cause another fission event.
2.3.1. K = 1 --> Reaction is self-sustaining
2.3.2. K < 1 --> Sub-critical, reaction will stop
2.3.3. K > 1 --> super-critical, can result in runaway nuclear reaction.
2.4. Mass Distribution of fission fragments
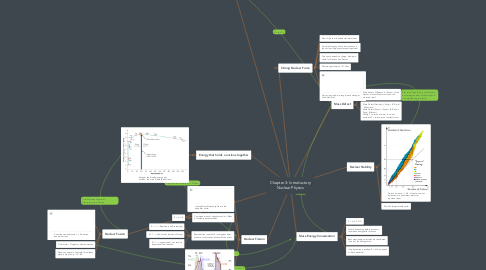
3. Binding Energy
3.1. Binding Energy(Nucleus) = Mass Defect x c^2
3.2. Binding energy per nucleon = Binding Energy of nucleus / Mass number of nucleus
4. Strong Nuclear Force
4.1. One of the four fundamental interactions
4.2. Force that binds protons and neutrons in the nucleus (Opposes electrical repulsion).
4.3. Does not depend on charge, binding is same for Neutron and Protons
4.4. Short range (order of 10^-15m)
4.5. Very strong within its range (much stronger than electrical).
5. Nuclear Fusion
5.1. Combine two light nuclei --> Produce a heavier nucleus
5.2. Final mass < Original combined masses
5.3. Need to overcome Coulomb force (takes place in high temp. ~10^8K
6. Mass Defect
6.1. Mass defect: Difference in the sum of the masses of constituent atoms and the molecule itself.
6.2. Mass Defect (Nucleus) = Zm(p) + (A-Z)m(n) - M(nucleus) Mass Defect (Atom) = Zm(p) + (A-Z)m(n) + Zm(e) - M(atom), Where Z = proton number, A = mass number, M = total mass of nucleus/atom.
7. Mass-Energy Conservation
7.1. E = mc^2 !!!!!!!
7.2. Total mass-energy before process = total mass-energy after process.
7.3. Rest mass-energy must also be considered in one of the energy forms
7.4. if a photon/ray is emitted, E = hf also needs to be considered.
8. Nuclear Stability
8.1. Protons increase --> No. of neutrons must increase more (counteract electrical repulsive force).
8.2. Pb is the largest stable nuclei



