1. Hair Introduction
1.1. Hair Structure **Hair is composed primarily of proteins (88%), these proteins are of a hard fibrous type known as keratin. **Hair is structured in three basic layers: Cuticle, Cortex and Medulla ***Hair Chemistry: ….. there are three types bond in the cortex layers of the hair: hydrogen bond, salt bond and cysteine bond. ***Hair Life Cycle: ……..There are three phases in hair life cycle: active growth phase, transition phase and resting phase
1.2. Life Cycle of the hair: Every hair follicle has a regular life cycle of growth, rest, and falling. It is normal and natural for up to 100 scalp hair to shed daily and approximately 100 other hair will appear in their place
1.2.1. Stages of hair Life Cycle: ……..There are three phases in hair life cycle: Anagen: The active growth phase, Catagen: Transition phase, and Telogen: Resting phase.
1.2.1.1. Stages of hair Life Cycle: Anagen (80-90%): The active growth phase, the hair cells at the base of the hair follicle are dividing repeatedly and the hair grows steadily. The length of this phase determine the maximum length that the hair will reach, and it varies from person to person. Catagen (10-15%): Transition phase: This phase last from two to four weeks, and is a transitional phase during which the hair stops growing. Telogen: Resting phase: During this period, the mechanism responsible for the replication of the cells at the base of the hair and a subsequent hair growth, are inactive for several months. By the end of telogen, the hair is only loosely attached to the follicle and can be easily pulled out simply by brushing or washing the hair…etc.
1.3. Hair has two parts:.
1.3.1. a) Hair root- Part of hair below the skin surface b) Hair shaft- Extends above the skin surface Structure of the Hair Shaft 1- Cuticle: •Outer layer of the hair, consist of overlapping scales. Provides the shine and feel of the hair, as well as protection. •Each scale is attached to the cortex. •Cuticles have the ability to open and close.
1.3.2. The Cortex •Cortex: is the middle layer of the hair...90% of the hair weight comes from the cortex. •Hair color pigment (melanin) is found in the cortex. •Physical and chemical changes in the hair take place in the cortex.
1.3.3. The Medulla: Medulla - Innermost part of the hair strand Composed of circular cells Some hair may lack a medulla, (fine, natural blondes) Little is known about the function of the medulla The medulla does not effect salon services
1.3.4. SIDE BONDS OF THE CORTEX 3 Different side bonds: -----Hydrogen. ---------------Salt. ---------------------Disulfide bonds. **Side bonds hold the keratin fibres and are responsible or the strength and elasticity of human hair. **Important for roller sets, thermal styling, perming and chemical relaxing
1.3.5. Chemical Composition of the Hair Hair is composed of protein, that grows from the cells in the hair follicle. As the protein grows, it keratinizes (hardens). When hair pushes its way through the skin, it is no longer alive. 90% protein, made up of amino acids, which make up elements.
1.3.6. SIDE BONDS OF THE CORTEX
1.3.6.1. Hydrogen Bond • Is a weak physical side bond •Easily broken by water or heat e.g. Wet set, (roller set)
1.3.6.2. Salt bounds Also weak, temporary side bond. •Easily broken by strong alkaline or acidic solutions. e.g. Some mild permanent waves
1.3.6.3. Disulfide Bond •Chemical side bond that is very different from the other two. •Are much stronger, far fewer of them. •Disulfide bonds joins to sulfur atoms to create a cysteine, give the hair its shape. •Not broken by heat or water. •Thio perms (thioglycollic acid) break disulfide bonds which are then reformed by thio neutralizers. •Hydroxide chemical hair, relaxers break disulfide bonds and they can never be reformed.
1.4. Hair Pigment All natural hair colour is the result of the pigment located within the cortex called Melanin. Melanin are tiny grains of pigment that give natural colour to hair. There are two different types of melanin: Eumelanin and Pheomelanin. Eumelanin: Provides brown and black colour to hair. Pheomelanin: Provides colours ranging from red and ginger to yellow/blonde tones.
1.4.1. Dry Hair And Scalp ---Can be caused by inactive sebaceous gland, and aggravated by excessive shampooing or dry air, such as during winter or in a desert climate. **Lack of sebum leads to hair that appears dull, dry and lifeless **Should be treated with products that contain moisturizers and emollients. **Frequent shampooing should be avoided, along with the use of strong soaps or products with a high alcohol content.
1.4.2. Oily Hair And Scalp Caused by improper shampooing or overactive sebaceous glands, and is characterized by a greasy build up on the scalp and an oily coating on the hair. -----Can be treated by properly washing with a normalizing shampoo. A well-balanced diet, exercise, regular shampooing, and good personal hygiene are essential to controlling oily hair and scalp.
1.5. Factors that Influence Hair Growth ---Diet ----Blood Circulation ------Emotion Disturbances --------Endocrine Glands ----------Medication ------------Heredity --------------Environment ----------------Products -----------------Appliances
1.6. Three basic components of hair soil: 1. Sebum itself, the oily secretion of the sebaceous glands. 2. Proteinaceous matter, originating from the cell debris of the stratum corneum layers of scalp skin, also the protein content of sweat. 3. Soil from the atmosphere and from hair care products.
2. Types of Shampoo Shampoos are of the following types Classification of shampoos based on the appearance): • Powder Shampoo. • Liquid Shampoo. • Lotion Shampoo. • Cream Shampoo. • Gel Shampoo. • Aerosol Shampoo. Specialized Shampoo (Classification based upon functionality): • Conditioning Shampoo. • Anti‐dandruff Shampoo. • Mild Baby Shampoo. • Acid balanced shampoo (pH 5.5).
3. Principles & Auxiliary Surfactants
3.1. Non-ionic detergents •Used as auxiliaries detergents. •Have sufficient cleansing but low foaming power.
3.1.1. Fatty acid alkanolamides (should not be used > 15%)
3.1.2. Poly alkoxylated derivatives
3.1.3. Amine oxides
3.2. Anionic Detergents: Metallic or alkanolamide salts of fatty acids (Soaps): mostly provided by saponification of vegetable oils and animal fats.
3.2.1. Alkyl SO4 (such as sodium lauryl SO4 (OSO-3):
3.2.2. Alkyl Ether Sulphate: Differ from alkyl SO4 by the addition of ethylene oxide molecule
3.2.2.1. Na lauryl sulphate
3.2.2.1.1. Poor foam
3.2.2.1.2. Foam need stabilizer
3.2.2.1.3. Relatively insoluble
3.2.2.1.4. Low price
3.2.2.2. Na lauryl ether sulphate
3.2.2.2.1. Copious foam
3.2.2.2.2. Stable foam
3.2.2.2.3. Good solubilizing power
3.2.2.2.4. High cost
3.3. Anionic Surfactants
3.3.1. Soaps Irritant, dull appearance In hard water causes deposition of Ca & Mg soap
3.3.2. Alkyl ether SO4 more sol. Good foaming thickening prop. With electrolyte non-irritant, (lauric & myristic) affected by hard water
3.3.3. Sulfosuccinates mild co-surfact. combined with (Aerosol OT) used in baby shampoo
3.3.4. Alkyl SO4 sod. Lauryl sulfate effective foaming, good cleansing, emulsifying & dissolving properties
3.3.5. Sulphonates Gp Alpha olefin sulfonates less irritant, higher solubilizing High foaming in presence of sebum Show hydrotropic properties, stable in acid & base
3.4. Cationic detergents: •The cleansing & foaming properties less than anionic surfactants. •Irritant to the eye. •Have strong affinity for proteins (keratin), so induce re- disposition of dirt on the fiber.
3.4.1. Amphoteric surfactants : •Have a quaternary N as well as anionic gp (carboxylic, sulfate or sulfonate). •At low pH behave like cationic, at high pH like anionic. •Good skin & mucous membrane compatibility, (WHY ?
3.5. Thickening Agents High viscosity is very important both for: product stability & handling. Electrolyte: normally Na OR NH4 CL OR sulfate ↑ viscosity by ↑ the size of micelle......large amount causes salting out. High molecular weight PEG 6000. Cellulose derivatives. Natural gum
3.6. Preservative Ensure that the products contain no pathogenic. Variety of preservatives are available: ⇒parabens. ⇒imidazolidinyl urea. ⇒glutaraldehyde. ⇒5-bromo-2-nitro-1,3-diol. ⇒sodium iodate. ⇒dimethyl dimethylol hydantoin.
3.7. Antioxidant Due to the presence of oxygen sensitive oils........shampoo need antioxidants such as ascorbic acid, tocopherol, butylhydroxyanisol.
3.8. Color Using color which meet USP specifications whenever possible. Color stability should be considered. Color fading can be minimize by adding a suitable uv absorber. Water soluble absorbers usually work best in shampoo. Benzophenone-4 and-2 (0.05-0.1%). Color should be added in solution, not as solid material. Aqueous solution color required preservative
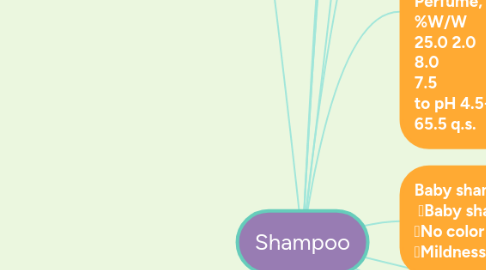
4. Presentative shampoo formulations: Clear lotion shampoo: %W/W Sodium Lauryl ether sulphate 45% (expensive so combine é SLS in ratio (1:2) Sodium Chloride (according to the viscosity required) 2-4% Perfume (1-5%) color, H2O, preservative to 100% Paste (opaque) shampoo: %W/W Sodium lauryl sulphate 33.0 Bentonite (clay) 8.0 Methyl cellulose 5.0 Deionized water 64.0 Perfume, preservatives and color q.s. Bentonite & Methyl cellulose are viscosity builders.
4.1. Opacifiers
4.1.1. Foam builders and stabilizers: A foam builder should be an ingredient which when added to a formulation, give the desired type of quantity of foam, e.g. non-ionic surfactants, amphoteric surfactants
4.1.2. pH The pH of a shampoo may have definite effects upon its properties: Most liquid shampoos today are formulated to have a pH between 6.5 and 8.5. Within this range a suitable viscosity and clarity can usually be achieved, as well as good stability and lathering properties. A few generalizations can be made concerning the effects o f pH on a typical clear shampoo formulation
4.1.3. Sequestring Agents Prevent the deposition of Ca & Mg soaps onto the hair when rinsing with hard water. Destabilize the membrane of the gram+ve organism which is stabilize by Ca & Mg ion, so making it ≥ permeable to preservatives.
5. Dandruff IS a common chronic scalp condition or disorder, which is marked by itching & flaking of the skin of the scalp. Not contagious. Or is shedding of dead skin cells from the scalp, which form flakes with oils, caused by frequent exposure to extreme heat & cold.
5.1. Causes
5.1.1. Dryskin
5.1.2. I Irritated, oily skin ( seborrhoeic dermatitis) red greasy skin covered by flaky white or yellow scales
5.1.3. Psoriasis, Eczema
5.1.4. Sensitivity to hair care products
5.1.5. not shampooing ↓ Sensitivity to hair care products ↓ Poor diet, lacks of zinc vit. B or certain types of fats enough, or shampooing too often, or using too many styling products
