Corrosion
by NBCS Engineering
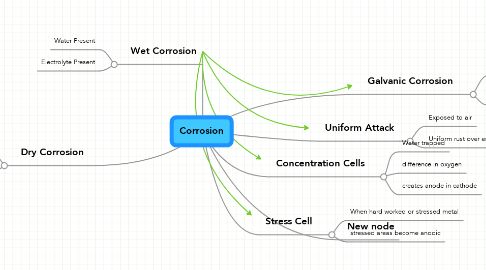
1. Wet Corrosion
1.1. Water Present
1.2. Electrolyte Present
2. Dry Corrosion
2.1. No water
2.2. High Temperature
3. Galvanic Corrosion
3.1. Presence of two dissimiliar metals
3.2. One metal more reactive than the other
4. Uniform Attack
4.1. Exposed to air
4.2. Uniform rust over entire surface
5. Concentration Cells
5.1. Water trapped
5.2. difference in oxygen
5.3. creates anode in cathode
6. Stress Cell
6.1. When hard worked or stressed metal
6.2. stressed areas become anodic
7. New node


