What we know
by Dominic Sung
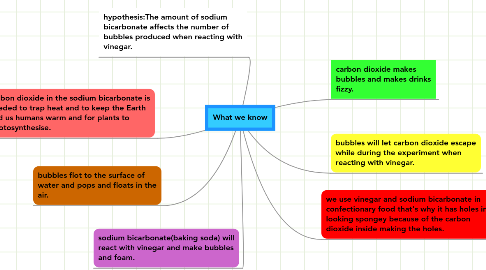
1. hypothesis:The amount of sodium bicarbonate affects the number of bubbles produced when reacting with vinegar.
2. carbon dioxide in the sodium bicarbonate is needed to trap heat and to keep the Earth and us humans warm and for plants to photosynthesise.
3. bubbles flot to the surface of water and pops and floats in the air.
4. sodium bicarbonate(baking soda) will react with vinegar and make bubbles and foam.
5. we use vinegar and sodium bicarbonate in confectionary food that's why it has holes in it looking spongey because of the carbon dioxide inside making the holes.
6. bubbles will let carbon dioxide escape while during the experiment when reacting with vinegar.
7. carbon dioxide makes bubbles and makes drinks fizzy.


