chemical Bonding
by olivia lam
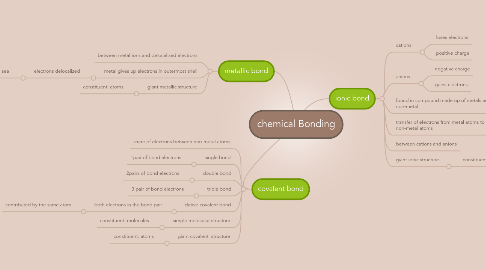

1. metallic bond
1.1. between metal ions and delocalized electrons
1.2. metal gives up electrons in outermost shell
1.2.1. electrons delocalized
1.2.1.1. forms electron sea
1.3. giant metallic structure
1.3.1. constituent: atoms
2. covalent bond
2.1. share of electrons between non-metal atoms
2.2. single bond
2.2.1. 1pair of bond electrons
2.3. double bond
2.3.1. 2pairs of bond electrons
2.4. triple bond
2.4.1. 3 pair of bond electrons
2.5. dative covalent bond
2.5.1. both electrons in the bond pair
2.5.1.1. contributed by the same atom
2.6. simple molecular structure
2.6.1. constituent: molecules
2.7. giant covalent structure
2.7.1. constituent: atoms
3. ionic bond
3.1. cations
3.1.1. loses electrons
3.1.2. positive charge
3.2. anions
3.2.1. negative charge
3.2.2. gains electrons
3.3. found in compound made up of metals and non-metal
3.4. transfer of electrons from metal atoms to non-metal atoms
3.5. between cations and anions
3.6. giant ionic structure
3.6.1. constituent: ions
