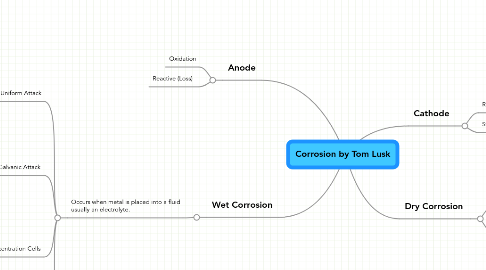
1. Anode
1.1. Oxidation
1.2. Reactive (Loss)
2. Wet Corrosion
2.1. Occurs when metal is placed into a fluid usually an electrolyte.
2.1.1. Uniform Attack
2.1.1.1. Some parts of metal will become anodic and others will become cathodic
2.1.1.2. Locations of the cathode and anode will continually change, resulting in uniform corrsion.
2.1.2. Galvanic Attack
2.1.2.1. Occurs when different metals are placed together in the presence of a corrosive environment.
2.1.2.2. Different metals have a greater affinity to corrode than others; one metal will become cathodic, while the other will become anodic
2.1.3. Concentration Cells
2.1.3.1. Occurs when there is a difference in concentration of the electrolyte.
2.1.3.2. Different oxygen levels in water create an anode part in the water and a cathode in the other part of the water.
2.1.4. Stress Cells
2.1.4.1. Result of high residual stress in parts of a metal object.
2.1.4.2. Areas of high stress tend to become anodic while the lower stress areas become cathodic.
