Factors Affecting Evaporation
저자: Dilip Aras
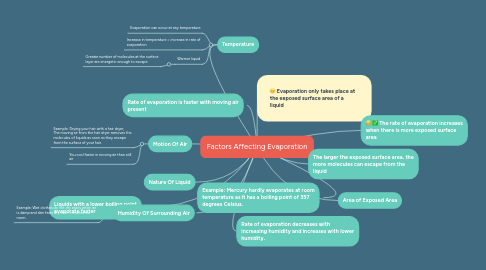
1. Temperature
1.1. Evaporation can occur at any temperature
1.2. Increase in temperature = increase in rate of evaporation
1.3. Warmer liquid
1.3.1. Greater number of molecules at the surface layer are energetic enough to escape
2. Rate of evaporation is faster with moving air present
3. Motion Of Air
3.1. Example: Drying your hair with a hair dryer, The moving air from the hair dryer removes the molecules of liquids as soon as they escape from the surface of your hair.
3.2. You cool faster in moving air than still air
4. Nature Of Liquid
5. Example: Mercury hardly evaporates at room temperature as it has a boiling point of 357 degrees Celsius.
6. Liquids with a lower boiling point evaporate faster
7. Humidity Of Surrounding Air
7.1. Example: Wet clothes do not dry easily when air is damp and skin feels dry in air-conditioned room.
8. Rate of evaporation decreases with increasing humidity and increases with lower humidity.
9. Evaporation only takes place at the exposed surface area of a liquid
10. The rate of evaporation increases when there is more exposed surface area
11. The larger the exposed surface area, the more molecules can escape from the liquid
12. Area of Exposed Area



