ACIDS, BASES AND BUFFERS CALCULATIONS
by Gemma Shearer
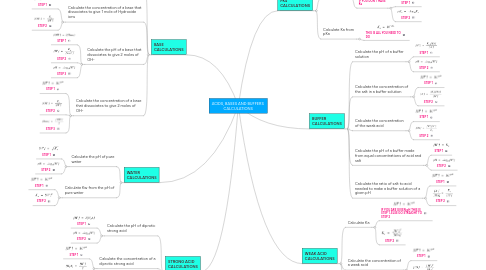

1. STRONG ACID CALCULATIONS
1.1. Calculate the pH of diprotic strong acid
1.1.1. STEP 1
1.1.2. STEP 2
1.2. Calculate the concentration of a diprotic strong acid
1.2.1. STEP 1
1.2.2. STEP 2
1.3. Calculate the pH of a monoprotic strong acid
1.3.1. THIS IS ALL YOU NEED TO DO
1.4. Calculate the concentration of s monoprotic Strong acid
1.4.1. THIS IS ALL YOU NEED TO DO
2. BASE CALCULATIONS
2.1. Calculate the pH of a base that dissociates to give 1 mole of Hydroxide ions
2.1.1. STEP 1
2.1.2. STEP 2
2.2. Calculate the concentration of a base that dissociates to give 1 mole of Hydroxide ions
2.2.1. STEP 1
2.2.2. STEP 2
2.3. Calculate the pH of a base that dissociates to give 2 moles of OH-
2.3.1. STEP 1
2.3.2. STEP 2
2.3.3. STEP 3
2.4. Calculate the concentration of a base that dissociates to give 2 moles of OH-
2.4.1. STEP 1
2.4.2. STEP 2
2.4.3. STEP 3
3. WATER CALCULATIONS
3.1. Calculate the pH of pure water
3.1.1. STEP 1
3.1.2. STEP 2
3.2. Calculate Kw from the pH of pure water
3.2.1. STEP 1
3.2.2. STEP 2
4. www.differentscience.com A level Biology and Chemistry tuition and resources
5. BUFFER CALCULATIONS
5.1. Calculate the pH of a buffer solution
5.1.1. STEP 1
5.1.2. STEP 2
5.2. Calculate the concentration of the salt in a buffer solution
5.2.1. STEP 1
5.2.2. STEP 2
5.3. Calculate the concentration of the weak acid
5.3.1. STEP 1
5.3.2. STEP 2
5.4. Calculate the pH of a buffer made from equal concentrations of acid and salt
5.4.1. STEP 1
5.4.2. STEP 2
5.5. Calculate the ratio of salt to acid needed to make a buffer solution of a given pH
5.5.1. STEP 1
5.5.2. STEP 2
6. WEAK ACID CALCULATIONS
6.1. Calculate Ka
6.1.1. IF YOU ARE GIVEN pH THIS IS STEP 1, ELSE GO STRAIGHT TO STEP 2
6.1.2. STEP 2
6.2. Calculate the concentration of a weak acid
6.2.1. STEP 1
6.2.2. STEP 2
6.3. Calculate the pH of a weak acid
6.3.1. STEP 1
6.3.2. STEP 2
7. PKa CALCULATIONS
7.1. Calculate pKa
7.1.1. IF YOU ARE GIVEN Ka
7.1.2. IF YOU DON'T HAVE Ka
7.1.2.1. STEP 1
7.1.2.2. STEP 2
7.2. Calculate Ka from pKa
7.2.1. THIS IS ALL YOU NEED TO DO
