Naming Chemical Bonds
par Kayla Nicole
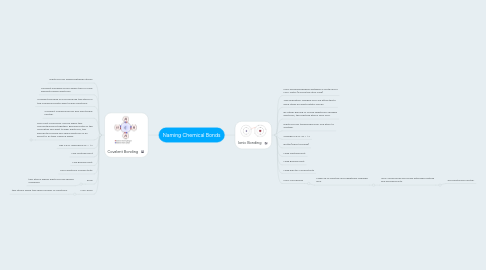

1. Covalent Bonding
1.1. Electrons are shared between atoms
1.2. Covalent bonding occurs when two or more elements share electrons.
1.3. Covalent bonding occurs because the atoms in the compound both seek to gain electrons.
1.4. Covalent compounds are also electrically neutral.
1.5. This most commonly occurs when two Nonmetals bond together. Because both of the nonmetals will want to gain electrons, the elements involved will share electrons in an effort to fill their valence shells.
1.5.1. kkakak
1.6. Has a E.N. difference of < 1.7
1.7. Low Melting Point
1.8. Low Boiling Point
1.9. Poor electrical conductivity
1.10. Polar
1.10.1. two atoms where electrons are shared unequally
1.11. Non-Polar
1.11.1. two atoms share the same number of electrons
2. Ionic Bonding
2.1. Ionic bonding happens between a Metal and a Non-Metal (Across the stair case)
2.2. The oppositely charged ions are attracted to each other by electrostatic forces.
2.3. By either gaining or losing negatively charged electrons, the reacting atoms form ions.
2.4. Electrons are transferred from one atom to another.
2.5. Change in E.N. of > 1.7
2.6. Brittle (Easy to break)
2.7. High Melting Point
2.8. High Boiling Point
2.9. High electric conductivity
2.10. Ionic Componds
2.10.1. Made up of positive and negatively charged ions.
2.10.1.1. Ionic compounds are solids with high melting and boiling points.
2.10.1.1.1. Are electrically neutral.

