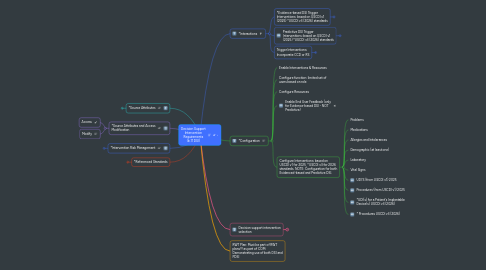
1. *Source Attributes
1.1. *Evidence-based
1.1.1. Bibliographic citation
1.1.2. Developer
1.1.3. Funding source
1.1.4. Release, rev date
1.1.5. Use of the patient demographics and observations data via USCDI standard
1.1.5.1. race
1.1.5.2. ethnicity
1.1.5.3. language
1.1.5.4. sexual orientation
1.1.5.5. gender identity
1.1.5.6. sex
1.1.5.7. date of birth
1.1.5.8. SDOH
1.1.5.9. Health status assessments data
1.2. Drug-drug and drug-allergy contraindication
1.2.1. Developer
1.2.2. Bibliographic citation
1.3. *Predictive Decision Support Interventions (PDSI)
1.3.1. Details and output of the intervention
1.3.2. Purpose of the intervention
1.3.3. Cautioned out-of-scope use of the intervention
1.3.4. Intervention development details and input features
1.3.5. Process used to ensure fairness in development of the intervention
1.3.6. External validation process,
1.3.7. Quantitative Measure
1.3.8. Ongoing maintenance
1.3.9. Update and continued validation or fairness assessment
1.4. *Enable limited set of users to author and revise source attributes (for both DSI and PDSI)
1.5. Must indicate when source attributes are not available (for 3rd party products)
2. *Source Attributes and Access Modification
2.1. Access
2.2. Modify
3. *Intervention Risk Management
3.1. Risk Analysis
3.2. Risk Mitigation
3.3. Governance
4. *Referenced Standards
4.1. *§ 170.213 - USCDI
4.2. § 170.299 - Incorporation by reference
4.3. § 170.102 - Definitions
5. *Interactions
5.1. *Evidence-based DSI Trigger Interventions: based on USCDI v1 (2025) *USCDI v3 (2026) standards
5.1.1. Problems
5.1.2. Medications
5.1.3. Allergies and Intolerances
5.1.4. Demographic (at least one)
5.1.5. Laboratory
5.1.6. Vital Signs
5.1.7. *UDI(s) for a Patient's Implantable Device(s)
5.1.8. *Procedures
5.2. Predictive DSI Trigger Interventions: based on USCDI v1 (2025) *USCDI v3 (2026) standards
5.2.1. Any USCDI element data
5.3. Trigger Interventions: Incorporate CCD or RS
5.3.1. Problems
5.3.2. Medications
5.3.3. Allergies & Intolerences
6. *Configuration
6.1. Enable Interventions & Resources
6.2. Configure function: limited set of users based on role
6.3. Configure Resources
6.4. Enable End User Feedback (only for Evidence-based DSI - NOT Predictive)
6.5. Configure Interventions: based on USCDI v1 for 2025, *USCDI v3 for 2026 standards. NOTE: Configuration for both Evidenced-based and Predictive DSI.
6.5.1. Problems
6.5.2. Medications
6.5.3. Allergies and Intolerances
6.5.4. Demographic (at least one)
6.5.5. Laboratory
6.5.6. Vital Signs
6.5.7. UDI'S (from USCDI v1) 2025
6.5.8. Procedures (from USCDI v1) 2025
6.5.9. *UDI(s) for a Patient's Implantable Device(s) USCDI v3 (2026)
6.5.10. * Procedures USCDI v3 (2026)
7. Decision support intervention selection
7.1. *Evidence-based DSI Interventions
7.1.1. Limited set of users (enabled/role)
7.1.2. in addition to drug-drug and drug-allergy contraindication checking
7.1.3. *Select/activate intervention using data expressed in these USCDI elements:
7.1.3.1. Problems
7.1.3.2. Medications
7.1.3.3. Allergies and Intolerances
7.1.3.4. Demographic (at least one)
7.1.3.5. Laboratory
7.1.3.6. Vital Signs
7.1.3.7. Procedures (from USCDIv1) 2025
7.1.3.8. *Procedures (USCDI v3 2026)
7.1.3.9. *UDI(s) for a Patient's Implantable Device(s) USCDI v3 2026
7.1.3.10. UDI(s) (from USCDI v1) 2025
7.1.4. Export End User intervention selection (ONLY FOR DSI, NOT PDSI)
7.2. Predictive DSI Interventions
7.2.1. Select/active intervention using data expressed in any USCDI elements
