Chemical Bonding
создатель sPARZTIC bOIBOI
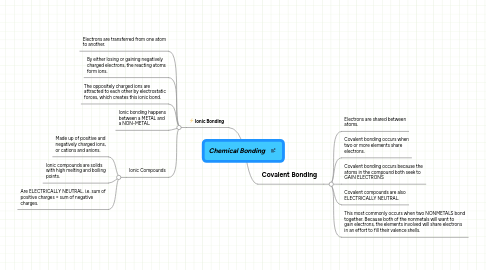

1. Ionic Bonding
1.1. Electrons are transferred from one atom to another.
1.2. By either losing or gaining negatively charged electrons, the reacting atoms form ions.
1.3. The oppositely charged ions are attracted to each other by electrostatic forces, which creates this ionic bond.
1.4. Ionic bonding happens between a METAL and a NON-METAL.
1.5. Ionic Compounds
1.5.1. Made up of positive and negatively charged ions, or cations and anions.
1.5.2. Ionic compounds are solids with high melting and boiling points.
1.5.3. Are ELECTRICALLY NEUTRAL. i.e. sum of positive charges = sum of negative charges.
