Matter
da Jacob Peters
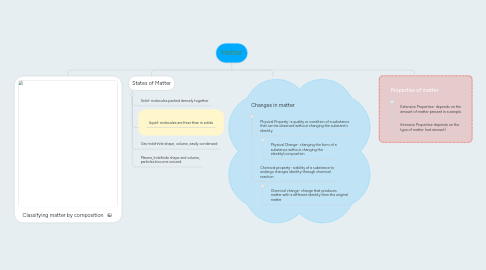

1. Classifying matter by composition
2. States of Matter
2.1. Solid- molecules packed densely together
2.2. liquid- molecules are freer than in solids
2.3. Gas-indefinite shape, volume, easily condensed
2.4. Plasma_Indefinite shape and volume, particles become ionized.
3. Changes in matter
3.1. Physical Property- a quality or condition of a substance that can be observed without changing the substane's identity
3.1.1. Physical Change- changing the form of a substance without changing the identity(composition
3.2. Chemical property- a ability of a substance to undergo changes identity through chemical reaction
3.2.1. Chemical change- change that produces matter with a different identity then the original matter
