1. Functional groups
1.1. Carboxylic Acids - formula CnH2nCOOH
1.1.1. Properties
1.1.1.1. Soluble in water
1.1.1.2. Polar
1.1.1.3. High melting/boiling points
1.1.1.4. Reeking odor
1.1.2. Contains a hydroxyl and carbonyl group.
1.1.3. Reactions with alcohol - esterification
1.1.4. IUPAC name: -oic acid
1.1.4.1. Functional group:
1.1.4.2. Always drop the 'e' suffix in root compound and replace it with IUPAC name.
1.2. Esters- formular CnH2n+2O
1.2.1. Properties
1.2.1.1. Polar
1.2.1.2. Usually soluble in water
1.2.1.3. Melting/boiling points less than alcohols
1.2.1.4. Very flammable
1.2.2. Esterification: Esters are formed by condensation reactions of a carboxylic acid and alcohol
1.2.2.1. Reactions involving Carboxylic acids and Esters
1.2.3. Hydrolysis: Reverse reaction of esterification by the addition of hydrogen and oxygen
1.2.4. IUPAC name: R'oxyR
1.2.4.1. To name a ester, it must be identified what the alcohol is. The -ol is dropped and -yl is added. For the carboxylic acid, the -oic acid is dropped and repleaced with -oate.
1.2.4.1.1. Methanol is the alcohol, and butanoic acid is the carboxylic acid. Therefore the resulting name is methyl butanoate.
1.3. Alcohols - formula CnH2nOH
1.3.1. physical properties
1.3.1.1. Polar
1.3.1.2. Soluble in water
1.3.1.3. Very flammable
1.3.1.4. melting/boiling points higher than alkanes
1.3.2. reactions
1.3.2.1. Combustion: alcohols + oxygen -> carbon dioxide and water
1.3.2.2. esterification: alcohol + carboxylic acid ->
1.3.2.3. Esters can be produced from the condensation reaction of alcohols
1.3.2.4. Can be produced by the hydration reaction of a alkene.
1.3.2.5. Can be reversed by a dehydration reaction to produce a alkene and water
1.3.3. polimerization
1.3.3.1. addition - breaking of C=C bonds in smaller alkene compounds
1.3.3.2. condensation - joining small monomer molecules by elimination
1.3.4. Types
1.3.4.1. Primary Alcohol (1°)
1.3.4.1.1. Hydroxyl group (-OH)l is bonded to a carbon atom at the end of the chain
1.3.4.2. Secondary Alcohol (2°)
1.3.4.2.1. Hydroxyl group (-OH) is bonded to a carbon atom that is attached to 2 other carbon atoms
1.3.4.3. Tertirary Alcohol (3°)
1.3.4.3.1. Hydroxyl group (-OH) is bonded to a carbon atom that has three alkyl groups bonded to it
1.4. Ethers- formula: CnH2n+2O
1.4.1. an organic compound that contains oxygen between 2 carbon atoms in a chain
1.4.2. Properties
1.4.2.1. Polar
1.4.2.2. Usually soluble in water
1.4.2.3. Melting/boiling points are lesser than alcohols
1.4.2.4. Very flammable
1.4.3. Naming
1.4.3.1. IUPAC name: R'oxyR
1.4.3.2. Add suffix '-oxy' to the smaller hydrocarbon group bonded to the larger alkane group.
1.4.4. Reactions
1.4.4.1. Ethers are produced by condensation reactions of 2 alcohols.
1.5. Amines - formula: R'amine
1.5.1. Subcategories
1.5.1.1. 1° Amine
1.5.1.1.1. Only 1 alkyl group is attached to nitrogen atoms
1.5.1.2. 2° Amine
1.5.1.2.1. Only 2 alkyl groups are attached to nitrogen atoms
1.5.1.3. 3° Amine
1.5.1.3.1. All 3 alkyl groups are attached to nitrogen atoms
1.5.2. Properies
1.5.2.1. Usually polar
1.5.2.2. Soluble in water
1.5.2.3. Strong fishy odours
1.5.2.4. Melting and boiling points decrease as more alkyl groups are attached to the nitrogen atom.
1.5.3. Naming
1.5.3.1. IUPAC name: RylR'ylR''ylamine
1.5.3.2. Always remove the '-e' from the ending and given the '-amine' suffix.
1.5.3.2.1. This is Ethanamine. It is a primary (1° Amine)
1.6. Amides- formula: R'amide
1.6.1. Properies
1.6.1.1. Polar
1.6.1.2. Smaller amides are soluble in water due to the N-H bonds that undergo hydrogen bonding
1.6.1.3. Melting and boiling point is higher than acids
1.6.2. Structure
1.6.2.1. Contains a carbonyl group attached to a nitrogen atom
1.6.3. Reactions
1.6.3.1. Condensation reactions of a carboxylic acid with ammonia or a primary and secondary amine can form a amide.
1.6.4. Naming
1.6.4.1. IUPAC name: RylR'ylR''ylamide
1.6.4.2. When naming the resulting ester, the first part comes from the amine, second part comes from the acid and the ending always contains the suffix '-amide'
1.6.4.2.1. The first part of this compound came from butanoic acid. The second part came from methanamine. Therefore the name is N-methylbutanamide
1.7. Carbonyl group
1.7.1. Aldehydes
1.7.1.1. contains a carbonyl group that is bonded to at least 1 hydrogen atom
1.7.1.2. Properties
1.7.1.2.1. Polar
1.7.1.2.2. Soluble in water
1.7.1.2.3. Melting/boiling points are higher than alkanes but lesser than alcohols
1.7.1.2.4. Pungent smells
1.7.1.3. Naming
1.7.1.3.1. IUPAC name: -al
1.7.1.3.2. Replace the final '-e' from the name of the parent alkane with the suffix '-al'
1.7.2. Reactions
1.7.2.1. Both aldehydes and ketones can be created through controlled oxidation of alcohol.
1.7.2.1.1. Controlled oxidization of a primary alcohol produces a aldehyde
1.7.2.1.2. Controlled oxidization of a secondary alcohol produces a ketone
1.7.2.1.3. Controlled oxidization of a tertiary alcohol does not oxidize.
1.7.2.2. Both aldehydes and ketones can produce alcohols through hydrogenation
1.7.2.2.1. Hydrogenation of a aldehyde can produce and primary alcohol
1.7.2.2.2. Hydrogenation of a ketone can produce an secondary alcholol
1.7.3. Ketones
1.7.3.1. contains a carbonyl group that is bonded to 2 carbon atoms
1.7.3.2. Properties
1.7.3.2.1. Polar
1.7.3.2.2. Soluble in water
1.7.3.2.3. Melting/boiling points are higher than alkanes but lesser than alcohols
1.7.3.2.4. Sweet smells
1.7.3.3. Naming
1.7.3.3.1. IUPAC name: '-one'
1.7.3.3.2. Replace the final '-e' of the parent alkane is replaced with the suffix '-one'
2. Hydrocarbons
2.1. Aromatic Compounds
2.1.1. Benzene- formula C6H6
2.1.1.1. Formula C6H6
2.1.1.2. Structure
2.1.1.2.1. Carbon to carbon bonds are all the same length
2.1.1.2.2. Flat 6-carbon ring with a hydrogen atom bonded to each carbon atom.
2.1.1.2.3. Simplest aromatic hydrocarbon
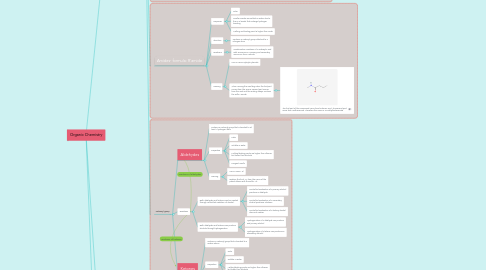
2.1.1.2.4. This is Benzene. Its distinct flat 6-carbon ring is shown bonded with a hydrogen atom for every carbon atom.
2.1.1.3. Properties
2.1.1.3.1. Liquid at room temperature
2.1.1.3.2. Crystalline solids
2.1.1.3.3. Generally insoluble in water
2.1.1.3.4. Higher melting/boiling points than alkynes.
2.2. Aliphatic Compounds
2.2.1. Alkenes - formula CnH2n
2.2.1.1. Higher melting/boiling points than alkanes.
2.2.1.2. can undergo addition reactions
2.2.1.2.1. Hydrogenation
2.2.1.2.2. hydration -> alcohols
2.2.1.3. others: functional group C=C
2.2.1.4. unsaturated compounds
2.2.1.5. IUPAC name: -ene
2.2.1.5.1. This is ethene. Ethene is an alkene because it has a carbon to carbon double bond.
2.2.2. Alkanes - formula: CnH2n+2
2.2.2.1. Saturated hydrocarbon
2.2.2.2. Insoluble in water
2.2.2.3. Soluble in organic solvents
2.2.2.4. Nonpolar
2.2.2.5. Saturated compounds that cannot undergo further adddition reaction, only substitution reactions
2.2.2.6. IUPAC name: -ane
2.2.2.6.1. This is ethane. Ethane is an alkane because it has a carbon to carbon single bond
2.2.2.7. Cyclic Alkane
2.2.2.7.1. Hydrocarbon in which structure consists of carbon atoms joined to form a closed rings
2.2.2.7.2. Structure
2.2.3. Alkynes- formula: Cn H2n-2
2.2.3.1. Unsaturated hydrocarbons
2.2.3.2. It contains at least 2 carbons bonded together by a triple bond
2.2.3.3. Nonpolar
2.2.3.4. Insoluble in water
2.2.3.5. Higher melting/boiling points than alkenes.
2.2.3.6. Undergoes addition reactions.
2.2.3.6.1. Hydrogenation
2.2.3.6.2. Halogenation
2.2.3.6.3. Hydration
2.2.3.7. IUPAC name: -yne
2.2.3.7.1. This is ethyne. Another name for ethyne is Acetylene. Acetylene is an alkyne because it has a carbon to carbon triple bond.
3. Naming organic compounds
3.1. identify longest carbon chain
3.1.1. 1 carbon = meth-
3.1.2. 2 carbons = eth-
3.1.3. 3 carbons = prop- .
3.2. identify type of bonding in the chain or ring
3.2.1. single-
3.2.2. double-
3.2.3. triple-
3.2.4. cyclo-
