CHEMICAL BONDING
da honey kumar
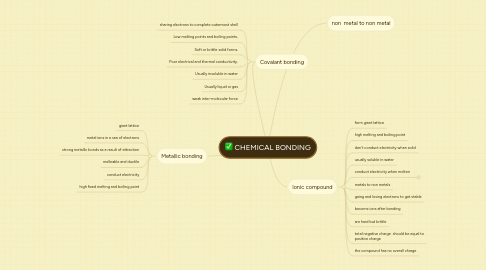

1. Covalant bonding
1.1. sharing electrons to complete outermost shell
1.2. Low melting points and boiling points.
1.3. Soft or brittle solid forms.
1.4. Poor electrical and thermal conductivity.
1.5. Usually insoluble in water
1.6. Usually liquid or gas
1.7. weak inter-molecular force
2. Metallic bonding
2.1. giant lattice
2.2. metal ions in a sea of electrons
2.3. strong metallic bonds as a result of attraction
2.4. melleable and ductile
2.5. conduct electricity
2.6. high fixed melting and boiling point
3. non metal to non metal
4. Ionic compound
4.1. form giant lattice
4.2. high melting and boiling point
4.3. don't conduct electricity when solid
4.4. usually soluble in water
4.5. conduct electricity when molten
4.5.1. t electricity
