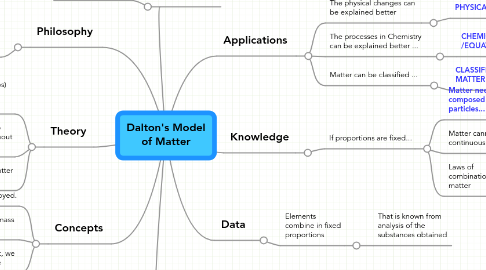
Dalton's Model of Matter
by Rafael Muñoa

1. Events Observed
1.1. Ratios in combination of elements
2. Focus Question
2.1. Is Matter continuous or discontinuous?
3. Data
3.1. Elements combine in fixed proportions
3.1.1. That is known from analysis of the substances obtained
4. Knowledge
4.1. If proportions are fixed...
4.1.1. Matter need to be composed of discrete particles...
4.1.1.1. ...called atoms
4.1.1.1.1. From here...
4.1.2. Matter cannot be continuous
4.1.2.1. Matter is discontinuous
4.1.3. Laws of combination of matter
5. Applications
5.1. The physical changes can be explained better
5.1.1. PHYSICAL CHANGES
5.2. The processes in Chemistry can be explained better ...
5.2.1. CHEMICAL REACTIONS /EQUATIONS
5.3. Matter can be classified ...
5.3.1. CLASSIFICATION OF MATTER
