Chemical Bonds
by alima sore
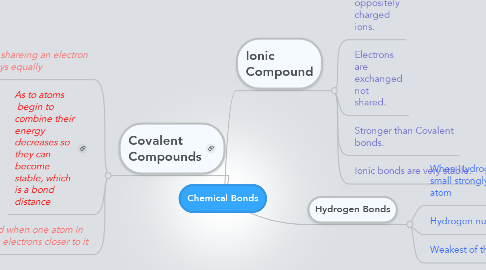
1. Covalent Compounds
1.1. they are shareing an electron not always equally
1.2. As to atoms begin to combine their energy decreases so they can become stable, which is a bond distance
1.3. Polar covalent Bond when one atom in particular is pulling electrons closer to it
2. Ionic Compound
2.1. It is a very strong bond between oppositely charged ions.
2.2. Electrons are exchanged not shared.
2.3. Stronger than Covalent bonds.
2.4. Ionic bonds are very stable.
3. Hydrogen Bonds
3.1. When Hydrogen bonds with small strongly electronegative atom
3.2. Hydrogen nucleus gains one proton.
3.3. Weakest of the bonds


