KInetic model of matter
by Malcolm Chow
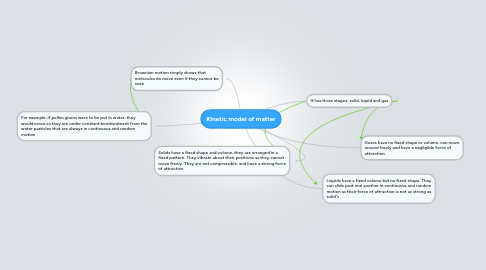
1. For example, if pollen grains were to be put in water, they would move as they are under constant bombardment from the water particles that are always in continuous and random motion
2. Brownian motion simply shows that molecules do move even if they cannot be seen
3. Solids have a fixed shape and volume, they are arranged in a fixed pattern. They vibrate about their positions as they cannot move freely. They are not compressible, and have a strong force of attraction
4. Gases have no fixed shape or volume, can move around freely and have a negligible force of attraction
5. Liquids have a fixed volume but no fixed shape. They can slide past one another in continuous and random motion as their force of attraction is not as strong as solid's
6. It has three stages: solid, liquid and gas


