Chemical Bonds
by Jenna Craven
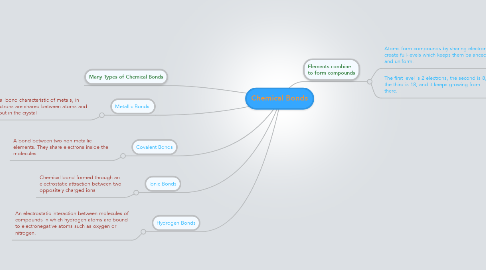
1. Metallic Bonds.
1.1. A chemical bond characteristic of metals, in which electrons are shared between atoms and move about in the crystal
2. Covalent Bonds
2.1. A bond between two non-metallic elements. They share electrons inside the molecules.
3. Ionic Bonds
3.1. Chemical bond formed through an electrostatic attraction between two oppositely charged ions
4. Hydrogen Bonds
4.1. An electrostatic interaction between molecules of compounds in which hydrogen atoms are bound to electronegative atoms such as oxygen or nitrogen.
5. Many Types of Chemical Bonds
6. Elements combine to form compounds
6.1. Atoms form compounds by sharing electrons to create full-levels which keeps them balanced and uniform.
6.2. The first level is 2 electrons, the second is 8, the third is 18, and it keeps growing from there.


