Gas
by Habib Farooq
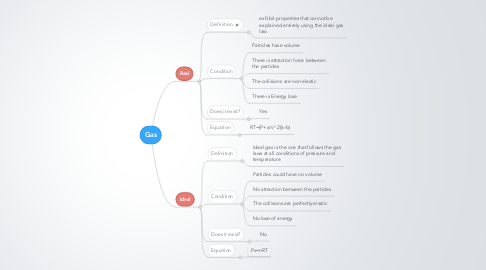

1. Real
1.1. Definition
1.1.1. exhibit properties that cannot be explained entirely using the ideal gas law.
1.2. Condition
1.2.1. Particles have volume
1.2.2. There is attraction force between the particles
1.2.3. The collisions are non-elastic
1.2.4. There is Energy lose
1.3. Does it exist?
1.3.1. Yes
1.4. Equation
1.4.1. RT=(P+a/v^2)(v-b)
2. Ideal
2.1. Definition
2.1.1. Ideal gas is the one that follows the gas laws at all conditions of pressure and temperature
2.2. Condition
2.2.1. Particles could have no volume
2.2.2. No attraction between the particles
2.2.3. The collisions are perfectly elastic
2.2.4. No lose of energy
2.3. Does it exist?
2.3.1. No
2.4. Equation
2.4.1. Pv=nRT

