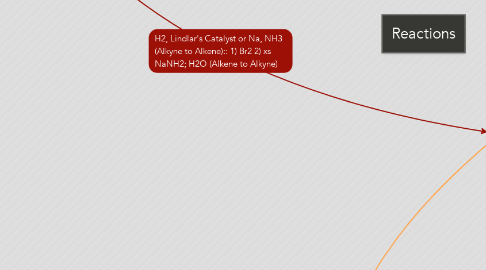
1. Alkynes
2. Ketones/Aldehydes
2.1. Hydrate Formation
2.2. Acetal Formation
2.3. Cyclic Acetal Formation
2.4. Cyclic Thioacetal Formation
2.4.1. Desulffurization
2.5. Imine Formation
2.6. Enamine Formation
2.7. Oxime Formation
2.8. Hydrazone Formation
2.9. Reduction of a Ketone
2.10. Grignard reaction
2.11. Cyanohydrin Formation
2.12. Wittig Reaction
2.13. Baeyer-Villiger Oxidation
2.14. Alpha Postion Reactions
2.14.1. Alpha Halogenation
2.14.1.1. Of Ketones
2.14.1.2. Of Carboxylic Acids
2.14.1.3. Haloform Reaction
2.14.2. Aldol Reactions
2.14.2.1. Aldol Addition and Condensation
2.14.2.2. Crossed Aldol Condensation
2.14.2.3. Intramolecular Aldol Condensation
2.14.3. Claisen Condensation
2.14.3.1. Claisen Condensation
2.14.3.2. Crossed Claisen Condensation
2.14.3.3. Intramolecular Claisen Condensation
2.14.4. Alkylation
2.14.4.1. Via Enolate Ions
2.14.4.2. The Malonic Ester Synthesis
2.14.4.3. The Acetoacetic Ester Synthesis
2.14.5. Michael Additions
2.14.5.1. Stabilized Carbon Nucleophiles
2.14.5.2. The Stork Enamine Synthesis
2.14.5.3. The Robinson Annulation
3. Acid Chlorides
4. Esters
5. Anhydrides
6. Amides
7. Nitriles
8. Amines
8.1. Preparation of Amines
8.1.1. From Alkyl Halides
8.1.1.1. 1)NaCN 2)xs LAH 3) H2O
8.1.2. From Carboxylic Acids
8.1.2.1. 1)SOCl2 2) xs NH3 3) xs LAH 4) H2O
8.1.3. From Benzene
8.1.3.1. 1) HNO3 H2SO4 2) H2 Pt or Fe H3O+
8.1.4. The Azide Synthesis
8.1.5. The Gabriel Synthesis
8.1.6. Via Reductive Amination
8.2. Reactions of Amines
8.2.1. Acylation
8.2.2. Hofmann Elimination
8.2.3. Reactions with Nitrous Acid
8.3. Reactions of Aryldiazonium Salts
8.3.1. Sandmeyer Reactions
8.3.2. Fluorination
8.3.3. Azo Coupling
8.3.4. Other Reactions

