Inside an Atom
by Spencer Witt
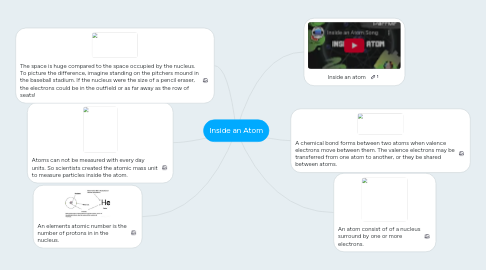
1. An elements atomic number is the number of protons in in the nucleus.
2. Atoms can not be measured with every day units. So scientists created the atomic mass unit to measure particles inside the atom.
3. The space is huge compared to the space occupied by the nucleus. To picture the difference, imagine standing on the pitchers mound in the baseball stadium. If the nucleus were the size of a pencil eraser, the electrons could be in the outfield or as far away as the row of seats!
4. An atom consist of of a nucleus surround by one or more electrons.
5. A chemical bond forms between two atoms when valence electrons move between them. The valence electrons may be transferred from one atom to another, or they be shared between atoms.
6. Inside an atom


