Corrosion
by Dan Sawyer
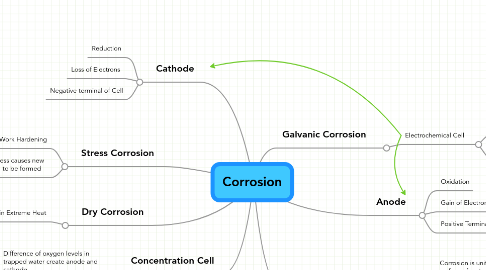

1. Stress Corrosion
1.1. Work Hardening
1.2. High stress causes new material to be formed
2. Cathode
2.1. Reduction
2.2. Loss of Electrons
2.3. Negative terminal of Cell
3. Dry Corrosion
3.1. Occurs in Extreme Heat
4. Concentration Cell
4.1. Difference of oxygen levels in trapped water create anode and cathode
5. Galvanic Corrosion
5.1. Electrochemical Cell
5.1.1. Electrolyte
5.1.1.1. Allows Electrons to flow from anode to cathode
5.1.2. Bridge
